A self-assembled composite of graphene oxide and chitosan can capture gold from electronic waste many times more efficiently and selectively than existing materials, researchers in Singapore have shown. The material could potentially eliminate several purification steps and make industrial recycling more economically competitive.
Because of the inertness of gold, its mining has a significant environmental footprint, usually requiring toxic chemicals such as mercury or cyanide to extract it from other components of the ore. This stability, together with its high electrical conductivity and ductility, makes it useful in electronics. Recycling gold to reduce mining faces the same extraction problems. Unwanted components are decomposed using one of several possible processes such as immersion in aqua regia (a concentrated mixture of hydrochloric acid and nitric acid). This yields a mixture of gold(I) and gold(III) ions mixed with copper, nickel, zinc and many others. ‘Now electrolysis is used,’ says Daria Andreeva at the National University of Singapore; ‘Electrolysis is a very long process that can take days or even weeks, so it’s a very interesting approach to look at how to make [separation] more efficient from an energy or time point of view.’

In previous work, Andreeva and colleagues led by Kostya Novoselov – who shared the 2010 Nobel prize in physics with Andre Geim for his work on graphene – developed self-assembled membranes from graphene oxide and other materials for applications ranging from tunable water filtration to corrosion prevention. In the new work, the researchers combined a solution of chitosan with a dispersion of graphene oxide flakes. When freeze dried, it formed a sponge-like material with ion-binding sites that could selectively capture and reduce both gold(I) and gold(III) ions – which must usually be extracted separately. Their material demonstrated substantially higher capacity to adsorb both ions: previous adsorbents have captured around 0.3g gold(I) and 2g gold(III) per gram of adsorbent – theirs captured 6.2g gold(I) and 16.8g gold(III).
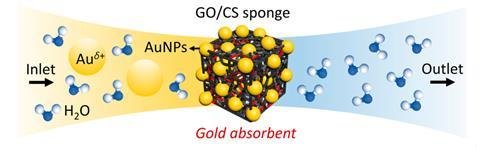
Moreover, their material did not require an electrical input – the material could donate sufficient electrons. ‘It’s a two-stage process,’ explains Novoselov. ‘The ions adsorb fairly tightly, but then we reduce them into the metallic state and we get nanocrystals of gold. These nanocrystals are not chemically adsorbed but only van der Waals adsorbed, so it is easier to remove them.’ The researchers are now working to develop a better understanding of the mechanism and to improve the specificity for gold still further. ‘In e-waste there is a thousand times more copper than there is gold,’ says Novoselov. ‘So while this is better than traditional materials, it’s still an open question whether it’s good enough for industry.’
‘This is really good,’ says Yang Su at Tsinghua University in China, who in 2022 co-authored a paper with Geim and others on gold extraction using reduced graphene oxide. ‘The adsorption capacity is astonishingly high.’ He notes the paper ‘shows us that there are a lot of things we didn’t know before about graphene systems and their chemistry’. He says he will be interested to see more detailed theoretical modelling of how the system can supply enough electrons to reduce so many gold cations with no applied voltage. He also hopes similar ideas might be useful in other metals, pointing out that if electric vehicles take over there will be a lot of unwanted platinum in catalytic converters to recycle.

