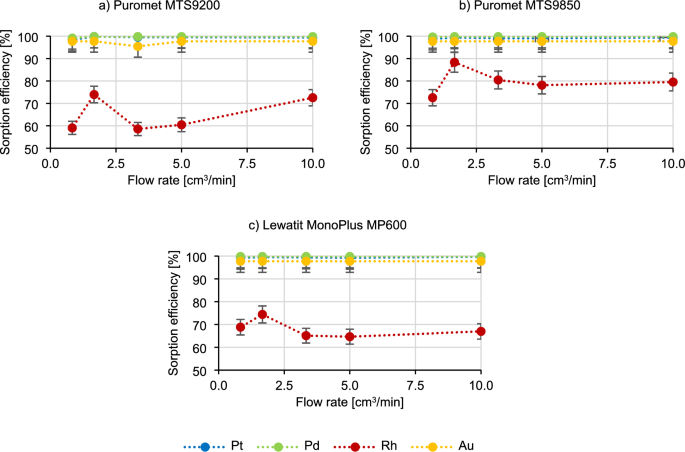
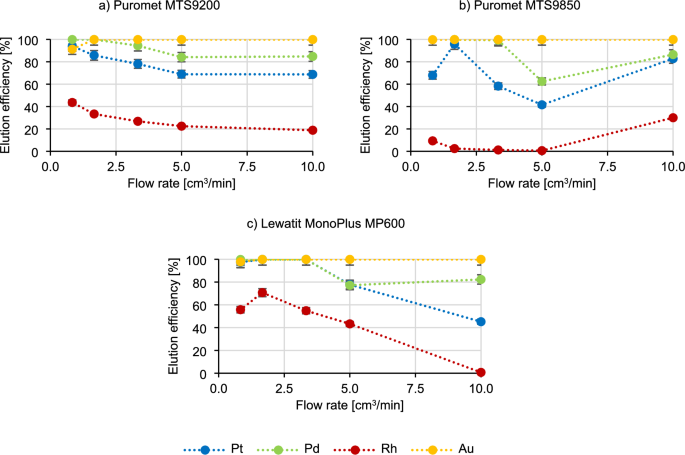
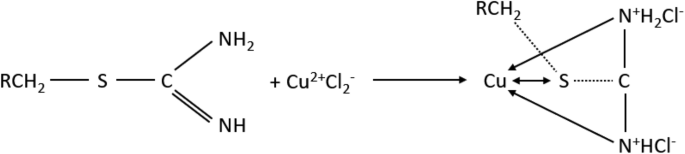The initial step in performing experiments under dynamic conditions involves verifying the contact time between a bed and a solution. These dynamic conditions simulate industrial settings, making it advantageous to explore the feasibility of shortening the process duration. Given the large scale of production in various industrial sites, it is often essential for a resin to efficiently adsorb elements within a brief timeframe. In the Fig. 1 the results of the impact of contact time on the course of the sorption process are present using three different resins.
Based on the results in Fig. 1, it can be said that the sorption efficiency of platinum, palladium and gold does not change with increasing the flow rate of the technological solution. Rhodium behaves most characteristically—in the beginning the sorption efficiency increases (for example, in the case of Puromet MTS9200 from 59.1% to 74.0%), then decreases rapidly (from 74.0% to 58.6% for Puromet MTS9200), which again has an increasing tendency again later on (to 72.6% for Puromet MTS9200). Picking the optimal flow rate in the industrial conditions is contingent on various interconnected processes. It is possible that sorption efficiency may improve at higher flow rates, however maintaining an excessively high flow rate is impractical. This is due to several factors, including the interplay between different processes that either supply materials for sorption or utilize them in the industry. Another consideration is equipment efficiency, as running equipment at maximum speed can result in higher energy costs. Moreover, the flow resistance of ion-exchange resins also poses a challenge, as over time, resins degradation and destruction increases flow resistance. Consequently, passing the solution through the bed at a higher flow rate could lead to significantly higher energy and operational costs, which is not optimal for industrial operations. Therefore, due to the high sorption efficiency of precious metals at the flow rate of 10 cm3/min (contact time 5 min), it was chosen as the basic parameter for further research.
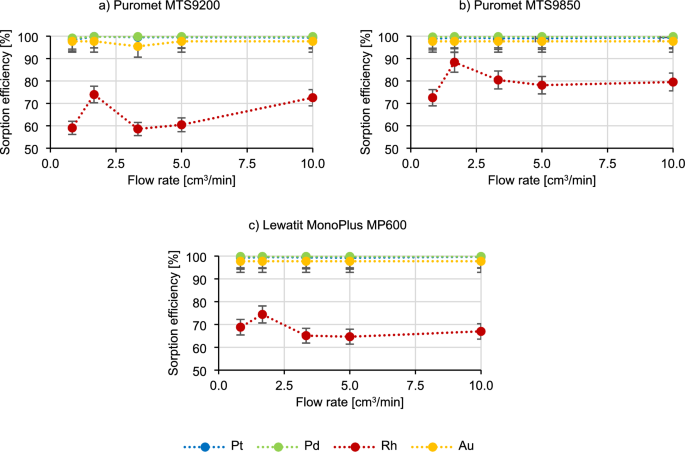
Another critical factor to assess under dynamic conditions is the bed geometry. Several resins may undergo volume changes in acidic or basic solutions, potentially impacting their operational efficiency. Therefore, it is essential to consider the optimal bed geometry when designing columns for future industrial applications. In the Fig. 2 the results of the impact of the bed geometry on the course of the sorption process are present.
The sorption efficiency of platinum, palladium and gold does not change with the difference in h/d, judging by the results presented in Fig. 2. The most specific obtained results are once again for rhodium, for which the sorption efficiency in the case of the Puromet MTS9200 remains at a similar level (about 70%), and after exceeding h/d ~ 6 it sharply drops (to 59.1%), in the case of using Puromet MTS9850, it remains at a similar level (about 76%), and in the case of using Lewatit MonoPlus, it first decreases with increasing h/d (from 67.0% to 55.3%), and after exceeding h /d ~ 6 increases rapidly (up to 70.2%). However, at the current level of research it is not possible to draw any specific conclusions and it can be assumed that the bed geometry does not influence the sorption efficiencies.
In the subsequent experiments, the impact of various factors (such as nitric acid, copper, and zinc concentration, and solution pH) on sorption efficiencies was investigated. These factors had been previously examined in static conditions, but it was deemed necessary to also evaluate them under dynamic conditions. As a result, only specific data points were retested in dynamic conditions to verify if the observed trends remained consistent52.
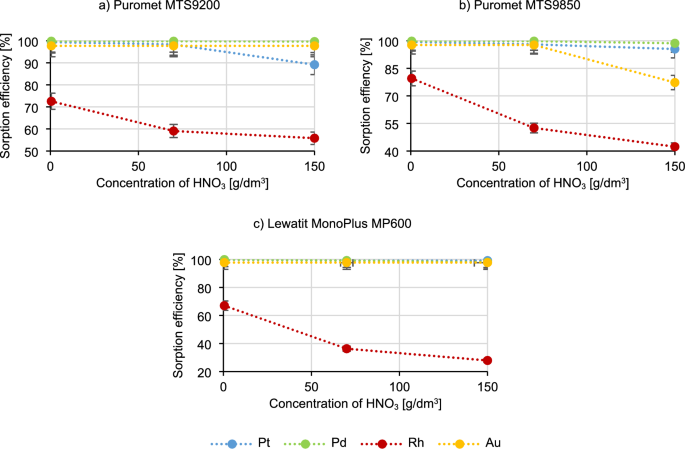
Figure 3 shows the results of the impact of the nitric acid concentration on the course of the sorption process.
Data included in Fig. 3 presents that as the concentration of nitric acid increases, the rhodium sorption efficiency decreases, in the case of Puromet MTS9200 to a level of approximately 55%, in the case of Puromet MTS9850 to 40%, and in the case of Lewatit MonoPlus MP600 to 30%. As the concentration of nitric acid increases, the platinum sorption efficiency for Puromet MTS9200 also decreases (from 98.4% to 89.2%), as for Puromet MTS9850 the gold sorption efficiency (from 97.7% to 77.3%) and also to a small extent platinum and palladium (change from 98.1% to 95.5% for Pt and from 99.8% to 98.7% for Pd). The visible decrease in the precious metals sorption efficiencies in the case of using Puromet MTS9850 is probably caused by the gel nature of this resin, which can degrade under the influence of the oxidizing conditions of nitric acid. Additionally, very acidic environment is not suitable for many resins due to the possibility of oxidation of functional groups.
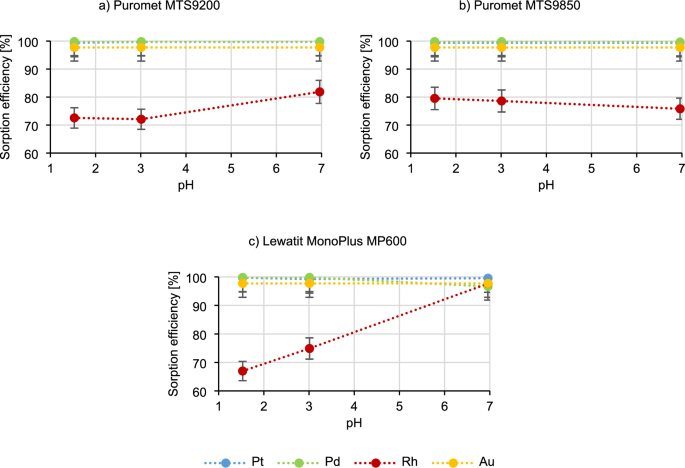
Figure 4 shows the results of tests on the influence of the pH of the technological solution on the sorption process.
In the case of Puromet MTS9200 and Puromet MTS9850, the sorption efficiency of platinum, palladium and gold does not change with the increasing pH. The rhodium sorption efficiency using Puromet MTS9200 increases (from 72.6% to 81.9%), and in the case of Puromet MTS9850 it decreases (from 79.5% to 75.8%). Distinctive results are observed using Lewatit MonoPlus MP600, where during the pH increase, the palladium sorption efficiency decreases (from 99.8% to 96.7%), but the rhodium sorption efficiency increases rapidly, reaching results even above 97%—at the pH of approximately 7. The same situation occurred when this parameter was tested in the static conditions. However why such situation occurs only while using one resin? The first explanation can be rhodium behaviour in the solutions with the increased pH. According to53 rhodium in the solutions with the pH above 2.9 undergoes hydrolysis according to a formula:
$$ \left[ {{\text{RhCl}}_{{{6} – {\text{n}}}} \left( {{\text{H}}_{{2}} {\text{O}}} \right)_{{\text{n}}} } \right]^{{{\text{n}} – {3}}} \to \, \left[ {{\text{RhCl}}_{{{6} – {\text{n}}}} \left( {{\text{H}}_{{2}} {\text{O}}} \right)_{{{\text{n}} – {1}}} {\text{OH}}} \right]^{{{\text{n}} – {1} – {3}}} + {\text{ H}}^{ + } $$
The new complex may have higher affinity towards the functional groups of the Lewatit MonoPlus MP600 resin (Quaternary ammonium type 2). Although it does not fully explain why such occurrence can be only observed for one resin. Among the three studied resins, Lewatit MonoPlus MP600 is the only one with strong base groups. According to34 it can be concluded that the distribution of Rh(III) drops significantly with the increase of chloride ion concentrations. Therefore when NaOH is added, Na+ ion combines with Cl– ion, creating a new stable compound. The concentration of free chloride ions in the solution drops and the distribution coefficient for Rh(III) increases. In weak base resins (Puromet MTS9200 and Puromet MTS9850) the distribution coefficient for rhodium is not influenced by the free chloride ion concentration. This means that to recover rhodium from technological solutions, Lewatit MonoPlus MP600 can be used, after prior neutralization of the solution.
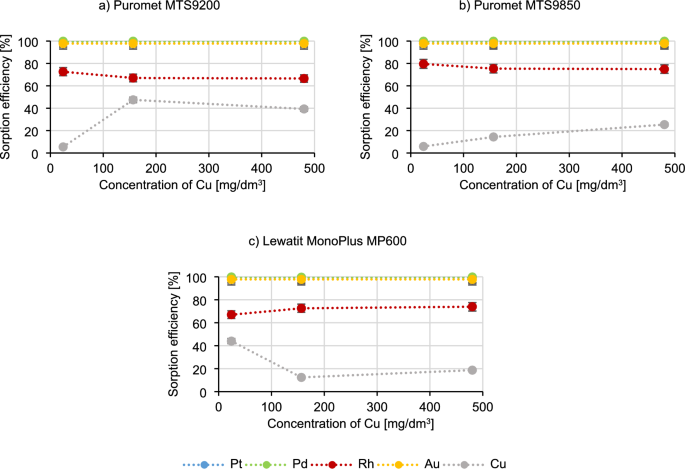
Figure 5 presents the results of researching the influence of copper concentration on the sorption process.
The graphs in Fig. 5 show that the sorption efficiencies of platinum, palladium and gold do not change with increasing copper concentration. When using Puromet MTS9200 and Puromet MTS9850, with an increase in copper concentration, its sorption efficiency also increases (for Puromet MTS9200 from 5.4% to 47.4%, and for Puromet MTS9850 from 5.8% to 25.3%) and at the same time the rhodium sorption efficiency decreases (by approximately 5% for both resins). A completely opposite situation occurs when Lewatit MonoPlus MP600 is used, where the copper sorption efficiency decreases (from 44.0% to 12.4%) and rhodium sorption efficiency increases (from 67.0% to 74.0%). This means that Puromet MTS9200 should not be used for solutions containing a high concentration of copper, because the copper ion may become a competitive ion in relation to the precious metal ions. Such situation is definitely problematic while dealing with precious metals solutions which often contain copper. According to54 copper belongs to a group of metals which exist in the form of equilibrating anionic species, therefore their behaviour is hard to predict:
$$ {\text{CuCl}}\left( {{\text{H}}_{{2}} {\text{O}}} \right)_{{3}}^{ + } \leftrightarrow {\text{ CuCl}}_{{2}} \left( {{\text{H}}_{{2}} {\text{O}}} \right)_{{2}} \leftrightarrow {\text{ CuCl}}_{{4}}^{{{2} – }} $$
Perhaps while adding Cu(II), more chloride complexes of Cu(II) are being formed which are competing with Pd(II) ions. Additionally Puromet MTS9200 is the only resin which contains isothiourionium functional groups—perhaps in the solutions with the higher concentrations of both copper and chloride ion (which was added with copper), Cu is starting to get higher affinity in comparison to the rest of precious metals as the stability constants of Cu–Cl complexes are lower than those of precious metals chlorocomplexes55. Additionally, copper has a high affinity for sulphur, which is also an important factor in the formation of the isothiuronium complex with Cu. In Fig. 6 a probable schematic reaction of the formation of Cu complex is presented56:
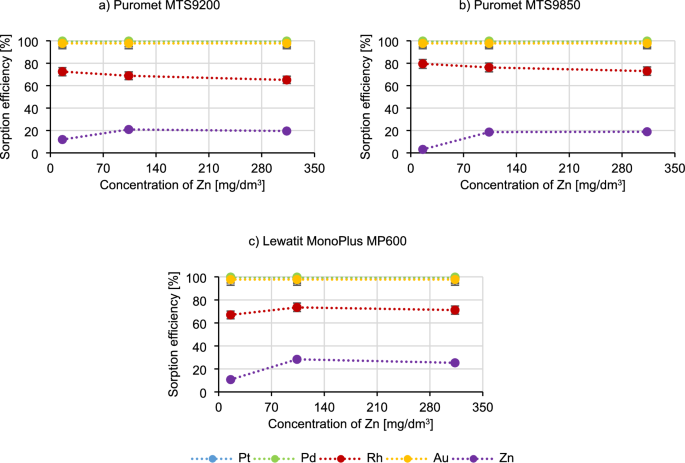
Figure 7 presents the results of testing the influence of zinc concentration on the sorption process.
Similarly, to the case of copper, when the zinc concentration increases, the sorption efficiency of metals such as platinum, palladium and gold does not change. The rhodium sorption efficiency when using Puromet MTS9200 and Puromet MTS9850 decreases, but only slightly (by about 6%), and in the case of Lewatit MonoPlus MP600, it can be said that the sorption efficiency practically does not change. The sorption efficiency of zinc increases as its concentration raises, but in all the tested conditions it did not exceed 30%. Based on the results, it can be concluded that Lewatit MonoPlus MP600 is not recommended for use for solutions containing high concentrations of zinc, because it may become a competitive ion towards precious metal ions. In the case of Zn the situation may be similar to Cu54. This metal is also in equilibrium with either neutral or cationic complexes:
$$ {\text{ZnCl}}\left( {{\text{H}}_{{2}} {\text{O}}} \right)_{{\text{x}}}^{ + } \, \leftrightarrow \,{\text{ZnCl}}_{{3}} \left( {{\text{H}}_{{2}} {\text{O}}} \right)_{{\text{y}}}^{ – } \, \leftrightarrow \,{\text{ZnCl}}_{{4}}^{ – } $$
Therefore, the same rationale used for copper can be applied to zinc as well. In addition, similar tests were conducted in a publication researching the sorption of platinum from the solutions containing Zn, Al, Fe, Cu and Ni. According to this article, [ZnCl4]2− is a competitive ion to [PtCl6]2− in the Pt microcomponent–ZnCl2 macrocomponent system, which is a situation which also is happening here, while adding additional amounts of Zn57.
In the previous static conditions tests, the impact of various parameters of the elution process on metal elution efficiencies was investigated. A solution containing 2 mol/dm3 of thiourea in 1 mol/dm3 of HCl was identified as the most effective eluting agent, with a volumetric ratio of Vr:Ve = 1:10 (Ve—volume of the eluting agent) deemed the most optimal. In the dynamic conditions these were used as the basic parameters to conduct the experiments. The influence of the contact time of the eluent with the bed on the elution process was researched and the results are presented in Fig. 8.
Based on the graphs in the Fig. 8, it can be deduced that gold elutes at a consistently high level (> 90%) at every flow rate. In the case of Puromet MTS9200, as the flow rate increases, the elution efficiency of each precious metal decreases (for Pt from 93.9% to 68.7%; for Pd from 99.9% to 84.1%; and for Rh from 43.7% to 18.9%). The same situation is visible for Lewatit MonoPlus MP600 (for Pt from 99.9% to 45.2%; for Pd from 99.9% to 82.3% to; and for Rh from 70.7% to 0.7%). In case of Puromet MTS9850 an decreasing tendency of elution efficiency of Pd (from to 99.8% to 83.4%) and Pt (from 99.9% to 41.6%) is visible. The elution of Rh is almost impossible while using Puromet MTS9850, although this metal can be later on recovered from the resin after elution by burning it in specials furnaces. This way obtaining concentrate containing only Rh is possible. The optimal elution results for each precious metal are obtained with the flow rates ranging from 1.67 to 5.00 cm3/min.
All experiments showed that rhodium was the most difficult element to recover due to its kinetically inert nature, which results in the largest fluctuation in its sorption efficiency58. As already mentioned, in acidic solutions, especially in aqueous chloride solutions, rhodium forms many complexes, such as [RhCl6]3−, [Rh(H2O)Cl5]2−, [Rh(H2O)2Cl4]− or [RhCl6]2−30,59. Although rhodium can occur in fourth oxidation state, it more often appears in chloride solutions in its third oxidation state, and undergoes solvation. This means that compounds with the H2O or OH ligands are formed. Precious metal complexes have a specific, already mentioned, tendency to form ion pairs with ion–exchange resin’s functional groups, according to the following series: [MCl6]2− > [MCl4]2− >> [MCl6]3− > aqua complexes. It shows that rhodium in the form of aqua complexes in the third oxidation state is the last to undergo the ion exchange process, which explains its low sorption efficiency and therefore largest fluctuation in its sorption efficiency.
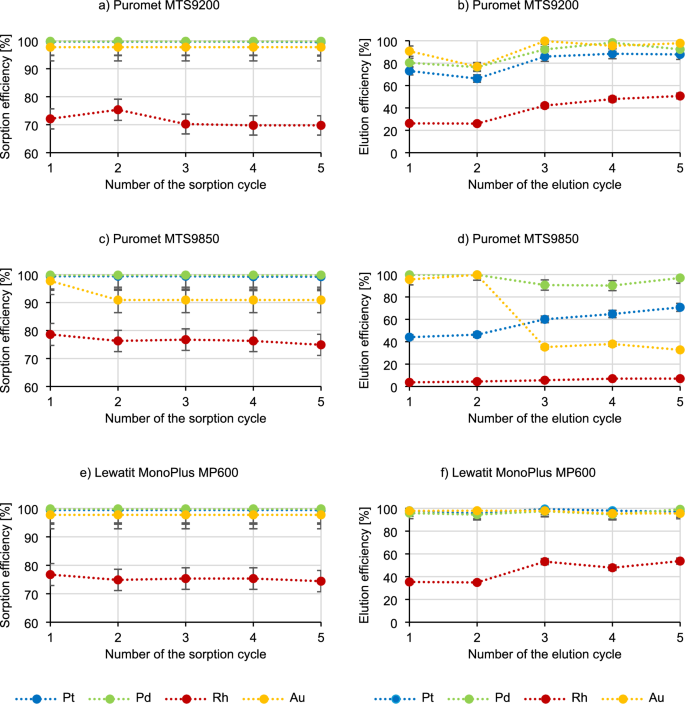
Figure 9 presents the results of five sorption–elution cycles.
Based on the analysis of the results presented in Fig. 9, it is visible that in the case of sorption using Puromet MTS9200, the sorption efficiency of precious metals remains at the same level. Using the same resin, the elution efficiency of precious metals increases with each successive elution cycle. In the case of sorption using Puromet MTS9850, there is a visible downward trend in the gold sorption efficiency (from 97.7% to 90.9%, already in the second cycle) and a low decrease in the sorption efficiency of rhodium and platinum (by 1–5%). In the elution process using this resin, there is also a visible decrease in the elution efficiency of gold (from 95.6% to 32.7%) and an increase in the elution efficiency of platinum (from 44.1% to 70.7%). In the case of Lewatit MonoPlus MP600, the efficiency of precious metals in the sorption process remains at a similar level as the number of cycles increases. Similarly, in the elution process, there is no change in the elution efficiency for platinum, palladium, and gold, and only increases for rhodium (from 35.4% to 53.7%).
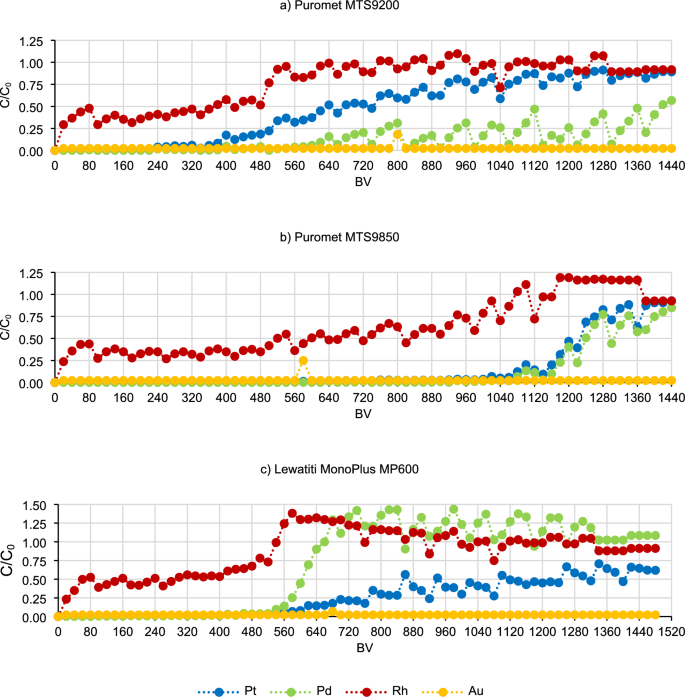
Subsequently, continuous sorption tests were carried out to explore the potential for achieving operational and total capacity of the resins. This investigation aimed to provide insights into the conditions under which ion exchange resins can operate most efficiently. The results of the continuous tests are presented in Fig. 10.
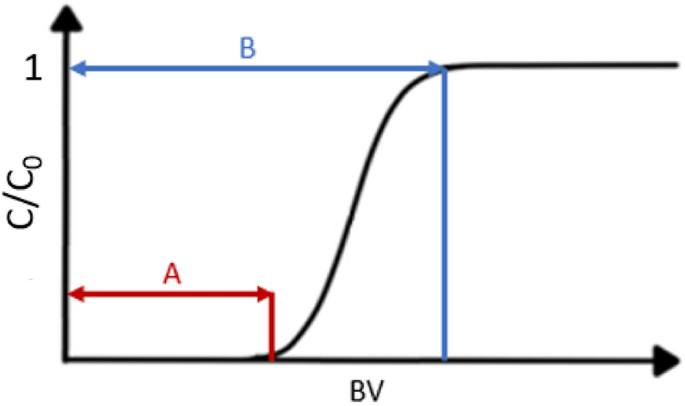
The obtained graphical curves indicate that the sorption process can be carried out for an even longer time, because not all points at which the operating and total capacity can be determined have been reached. However, based on the obtained results, the operating capacity for selected metals can be calculated—it is the first inflection point when the concentration of metal in the post-sorption solution begins to increase rapidly (line A in Fig. 11). The period before reaching the operating point determines the best working capacity of the column. In some cases, it was also possible to determine the total capacity—it is the point at which the metal concentration in the post-sorption solution equals the concentration in the pre-sorption solution dosed to the column (line B in Fig. 11). This is the moment when the resin will no longer adsorb the metal.
For specific resins, the operating and total capacities and their corresponding degrees of saturation were determined and summed in Table 3. The concentrations of metals in the post-sorption solutions after continuous sorption tests are presented in Tables SD-1–SD-3 in Supplementary Data. The data was utilized for plotting the curves, so all subsequent conclusions also pertain to the data presented in the Supplementary Data Tables.
Operating and total capacities are very important parameters while trying to scale up the process in dynamic conditions. As previously mentioned, operating capacity is the range when the column is working with the best efficiencies. In the industry, when this point is overpassed, the solution is then dosed to another column and the first one undergoes elution and regeneration step. In Table 3 it can be seen that the highest operating capacity was obtained for Puromet MTS9850 (for Pt and Pd 1060 BV, for Rh 980 BV), therefore in the industrial conditions this resin would be able to work for a longer time during one cycle than the other two tested resins. The operating capacities for different metals while using both Puromet MTS9850 and Lewatit MonoPlus MP600 are pretty similar, which allows to stop the process in one moment. The situation looks different while using Puromet MTS9200—it is almost impossible to assign one point in which the sorption should be stopped. In multicomponent solutions, especially while trying to recover more than one metal, it is better if most of the elements have similar operating capacities. The total capacity is a point when the resin cannot sorb more of the specific metal. For these tests, the threshold for reaching the total capacity was set at 0.9 instead of 1, taking into account the low levels of precious metals and potential analytical measurement errors of up to 10%. It is very important to mention that the total capacity and the ion-exchange capacity (in this case anion-exchange capacity) are not the same thing. Total capacity can be calculated for a specific solution and it will differ while using different one, while the ion-exchange capacity is the maximal amount of ions which can be sorbed by the resin.
In the case of gold, neither the operating capacity nor the total capacity could be achieved using these three ion exchange resins. The reason for this is probably the low concentration of gold in the initial solution and the high affinity of it to the ion exchange resins.
Microscopy photos of the ion exchange resins were also taken before conditioning and after continuous sorption experiments. The photos are shown in Fig. 11.
As can be seen in Fig. 12, Puromet MTS9200 contains beads of the most diverse sizes, while Lewatit MonoPlus MP600’s beads have very similar dimensions. A change in the colour of the ion-exchange resins after the sorption process is also visible, probably due to the sorption of precious metal complexes of different colours (from red to yellow). In no case were any significant defects such as chips, cracks or fractures observed.
In Table 4 a semi-quantitative analysis of the ion-exchange resin, before and after the sorption is presented.
The structure of ion-exchange resins such as Puromet MTS9850 and Lewatit MonoPlus MP600, which are composed of polystyrene crosslinked with divinylbenzene, was confirmed through semi-quantitative analysis data. These resins mainly consisted of carbon, with the incorporation of chlorine indicating the presence of vinyl chloride or a chloride radical added in the polymerization process. Puromet MTS98500, on the other hand, has a matrix of polyacrylate crosslinked with divinylbenzene, as shown by the high carbon and oxygen content. Despite undergoing sorption processes, the composition of ion-exchange resins remains largely unchanged, with any variations in elemental content attributed to the proportional appearance of precious metals.
It is important to note that there are currently no published studies addressing the calculation of resins capacities in dynamic conditions using industrial solutions. Only one publication describes the research conducted in dynamic conditions while using one of the same ion-exchange resins (Puromet MTS9850)46. The comparison is included in Table 5.
This research represents a novel approach in the scientific community for recovering precious metals through ion-exchange method using waste of the refining process. It marks a significant advancement towards industrial application and will greatly contribute to scaling the process in future studies.
Conducting a full economic study may be impossible at this point of research as the full technology for the production of pure precious metals from the refining waste has not been yet developed. However for comparative purposes, we can assume that at the beginning the possible installation can process 1 tonne of refining waste containing 0.05% of each precious metal (Pt, Pd, Rh, and Au). The recovery of precious metals is never complete, which is why the efficiency of the entire technology was assumed to be 99%. The market prices of individual precious metals as of May 13, 2024 (according to kitco.com60) were: for gold—EUR 69,825.42/kg; for platinum—EUR 29,721.25/kg; for palladium—EUR 28,975.98/kg; for rhodium—EUR 137,099.32/kg. This means that the installation can produce precious metals (with a purity of 99.9%) worth: 0.495 kg of gold—EUR 34,563.58; 0.495 kg of platinum—EUR 14,712.02; 0.495 kg for palladium—EUR 14,343.11; 0.495 kg for rhodium—EUR 67,864.16. Therefore, from 1 tonne of low-quality waste the possible installation can manufacture precious metals worth EUR 131,482.87.