AgNPs were successfully biosynthesized
The extraction of R. stricta leaves yielded a semi-solid dark green aqueous extract and its alkaline fraction (Dark brown) with yields of 40 g and 30.1g, respectively. Both preparations were light yellow when dissolved in distilled water for AgNPs synthesis. The biosynthesized AgNps displayed a dramatic serial color shift from bright yellow to yellow, brown, and eventually dark brown, indicating silver ion reduction and full production of stable silver ions. Various spectroscopy and microscopic examinations were used to characterize biosynthesized AgNPs.
AgNPs’ UV–visible spectra were measured between 200 and 900 nm. The UV–Vis spectra of Aq. AgNPs and Alkaline Aq. AgNPs resulted in two broad peaks at 405 and 415 nm, respectively (Fig. 1). As shown in Fig. 1, the peak of Alkaline Aq. AgNPs were higher than that of aqueous AgNPs, which might be attributed to increased energy consumption by the nanoparticles due to complicated bonding.
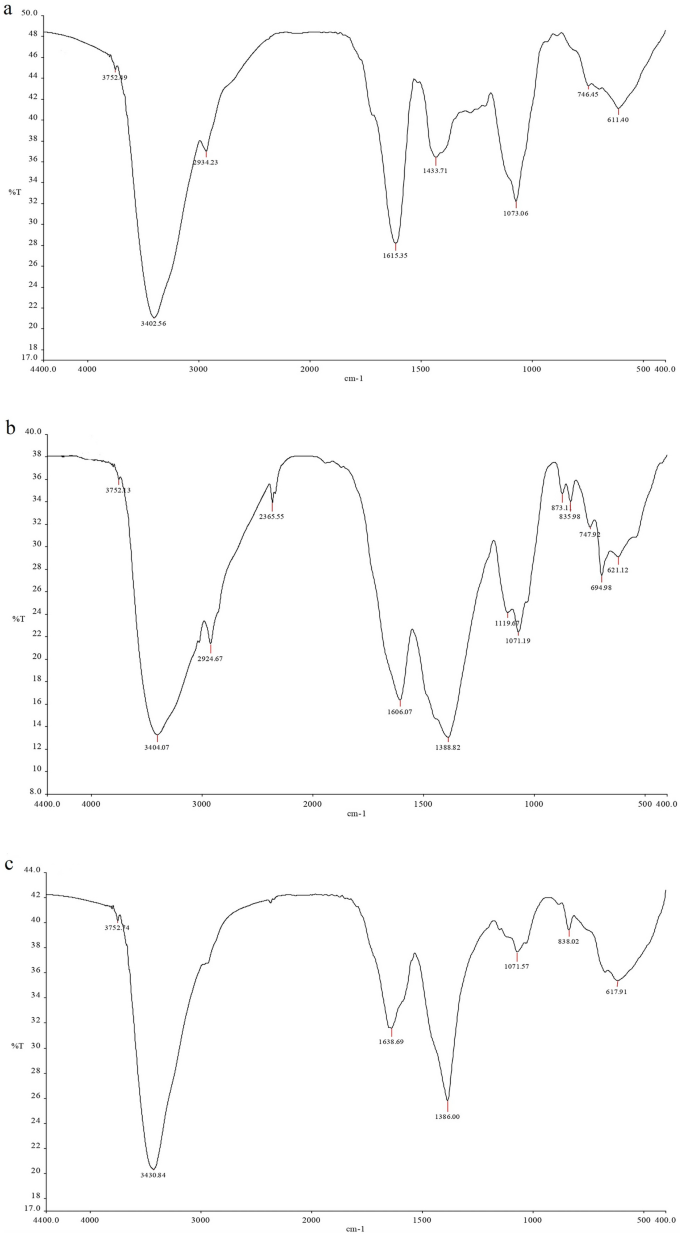
The FTIR spectra of R. stricta aqueous extract and biosynthesized AgNPs were analyzed to determine the nature of active ingredients responsible for nanoparticle reduction, stability, and bio-capping (Table 1 and Fig. 2). The presence of both bonded and non-bonded hydroxyl groups was indicated by broad peaks at 3402–3431 cm−1 and very weak peaks at 3402 cm-1 in the FTIR spectra of R. stricta preparations. Furthermore, all preparations showed peaks around 611–695 cm−1, indicating the existence of aliphatic bromo compounds. Methylene, aromatic rings, methyl, and cyclic ethers were found in both the crude extract and aq. AgNPs. The FTIR study of aqueous AgNPs indicated two distinct functional groups for nitrile (2365.55 cm−1) and secondary alcohol (1119.67 cm−1) compounds. At 836–897 cm−1, the aq. AgNPs and Alkaline Aq. AgNPs indicated the presence of peroxide. Table 1 shows that only the Alkaline Aq. AgNPs possessed four distinct functional groups for Quinone or conjugated ketone (at 1635.69 cm−1), sulfate (at 1386.00 cm−1), and amines (at 1242.06 and 1071.57 cm−1).
By calculating the velocity of the nano-sized particles, zeta potential analysis may be used to measure the surface charge and stability of the formulation of biosynthesized AgNPs. The velocity of nanoparticles is determined by their movement towards electrodes under the influence of an applied electric field. The Zeta potential of AgNPs produced by various R. stricta extracts and fractions was measured. The results showed that the A. AgNPs had a − 27.7 mV and the Alkaline Aq. AgNPs had a − 37.9 mV. These results demonstrated that the AgNPs produced are stable (Fig. 3). The average particle size, diameter, and polydispersity indices (PDI) of all pre-synthesized AgNPs, on the other hand, were evaluated. The average particle sizes (z-average) of Aq. AgNPs and Alkaline Aq. AgNPs were 95.9 nm (PDI value 0.220, intercept 0.874) and 54.04 nm (PDI value 0.464, intercept 0.829), respectively (Fig. 3). This shows a difference in particle size between the two preparations, which might be related to pH changes.
The two AgNPs preparations from R. stricta were analyzed by TEM imaging to corroborate the results of the zeta potential study. The findings revealed that both AgNPs were spherical in form, widely dispersed, and exhibited no aggregation (Fig. 4). The average diameter of Aq. AgNPs nanospheres ranged from 32 to 87 nm, whereas the average diameter of Alkaline Aq. AgNPs were 7.3–24.5 nm.
Fungistatic properties of R. stricta aqueous preparations
The antifungal effects of R. stricta aqueous extract and biosynthesized AgNPs against plant diseases such as D. halodes, D. tetramera, M. phaseolina, A. alternata, and C. australiensis were tested on a PDA medium. The suppression of mycelial growth was measured in all treatments and compared to positive and negative controls by measuring the diameter of clear zones in PDA dishes.
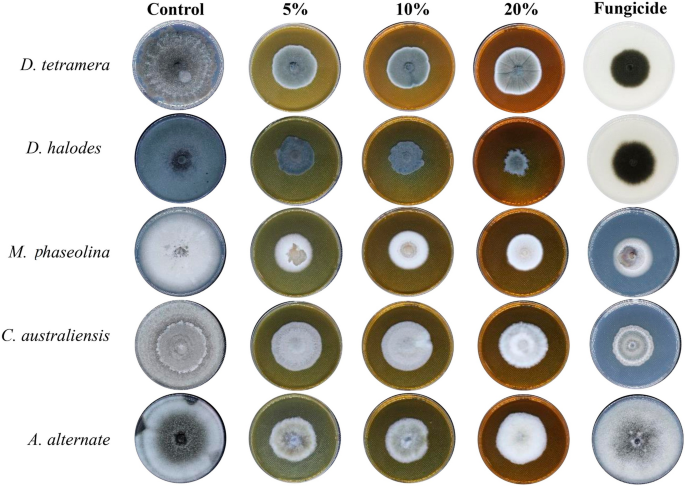
As shown in Fig. 5, the antifungal activity of the aqueous extract was at its maximum with a concentration of 20%. D. halodes was the most sensitive species to the fungicidal effects of all concentrations of R. stricta aqueous. On the other hand, C. australienses was the most resistant among all species (P < 0.001). Previcur energy had similar activities; however, it didn’t affect the mycelial growth of A. alternate (Fig. 5, Table 2).
Treatment with Aq. AgNPs showed stronger antifungal activities against all species. As shown in Fig. 6, all concentrations almost stopped the mycelial growth of all species compared to the negative control. The calculated percentages of the mycelial growth inhibition (IMG%) of A. alternata had the maximum IMG% (100%) followed by D. tetramera (95.9%), C. australiensis (93.4%), D. halodes (91.5%), and M. phaseolina (90.4%) at the 100% dose of Aq. AgNPs (P < 0.001) (Table 3).
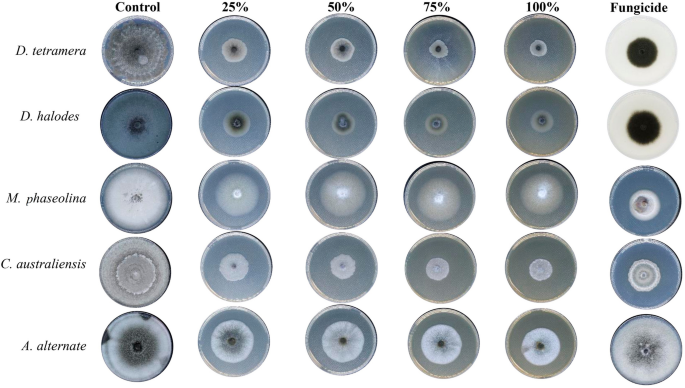
Treatment with Aq. AgNPs showed stronger antifungal activities against all species. As shown in Fig. 7, various concentrations had variable effects on the mycelial growth of all species compared to the negative control. The calculated IMG% was the maximum for D. halodes (86.4%), followed by D. tetramera (75.5%), C. australiensis (74.8%), A. alternata (54.2%), and M. phaseolina (46.2%) at the 100% dose of Aq. AgNPs (P < 0.001) (Table 4). A summary of the different antifungal effects of tested preparations of R. stricta is shown in Fig. 8. It was revealed that Aq. AgNPs were the most effective fungicide among all treatments, while the crude extract had the weakest antifungal effects except for M. phaseolina and A. alternata compared to the Alkaline Aq. AgNPs. Among all of the tested species, it seems like A. alternata was the most sensitive to the highest concentrations of Aq. AgNPs, while D. halodes was the most sensitive species to the treatment with the crude aqueous extract and Alkaline Aq. AgNPs of R. stricta. Controversially, M. phaseolina was statistically the most resistant to the inhibitory effects of all treatments, compared to the negative control.
Ultra-structural changes induced by AgNPs of R. stricta
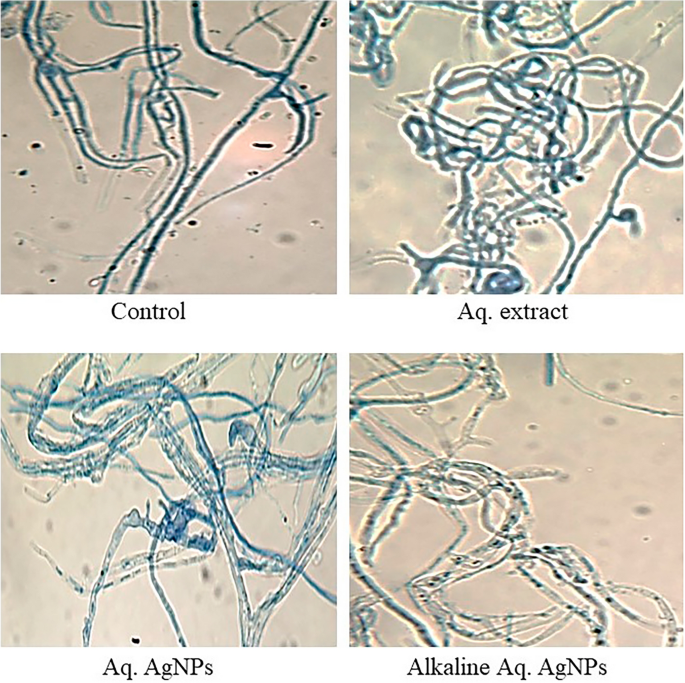
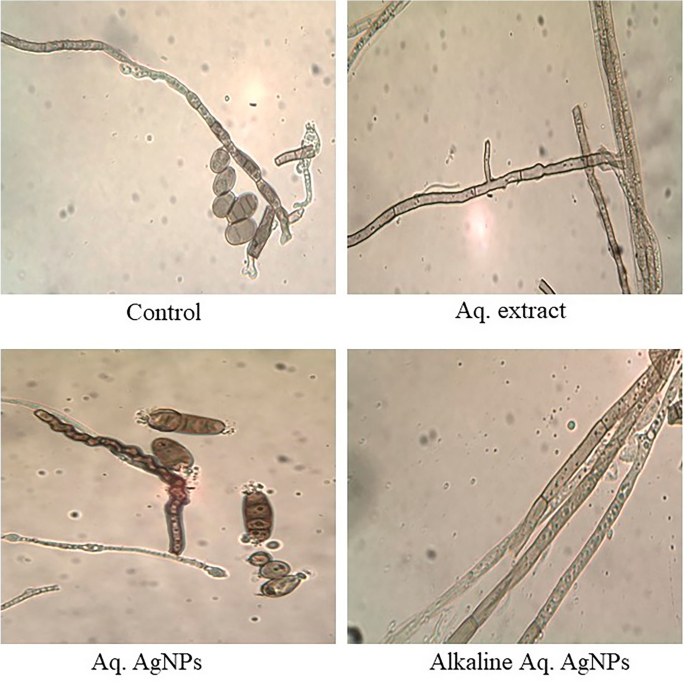
Light microscopy imaging was used in the current investigation to compare the morphological changes in treated samples to the untreated control. All investigated fungal strains showed variances in the natural shape and size of hyphae and conidiophores. The morphological analysis of untreated D. tetramera showed light brown conidiophores, semi-elongated ellipsoid, cylindrical, and with round to oval ends. They are septate with no more than three pseudoseptates. The hyphae were brownish, granulated, unbranched, and with slight winding walls. Treatments with various preparations of R. stricta affected the septation and cylindrical shape of conidiophores, which appeared more oval, darker, and less viable than the control. The hyphae looked smoother, zigzag-shaped, swollen (in the case of crude aqueous extract), semi-branched (in the case of biosynthesized AgNPs), and bale-brown in Aq. AgNPs treatment (Fig. 9).
The morphological analysis of untreated D. halodes showed the light brown, subcylindrical conidia with smooth, elongated walls. The geniculated conidiophores are septate transversally and have distinct septa at the basal cells with 6–8 pseudosepta. The hilum appeared distinctly protuberant and unbranched. Images of different treatments of R. stricta showed the conidia darker and less septate than in the control. The hyphae looked smoother, vacuolated, swollen, and semi-branched (in the case of crude biosynthesized AgNPs), budding, thicker, and bale-brown (in the case of Alkaline Aq. AgNPs treatment) (Fig. 10).
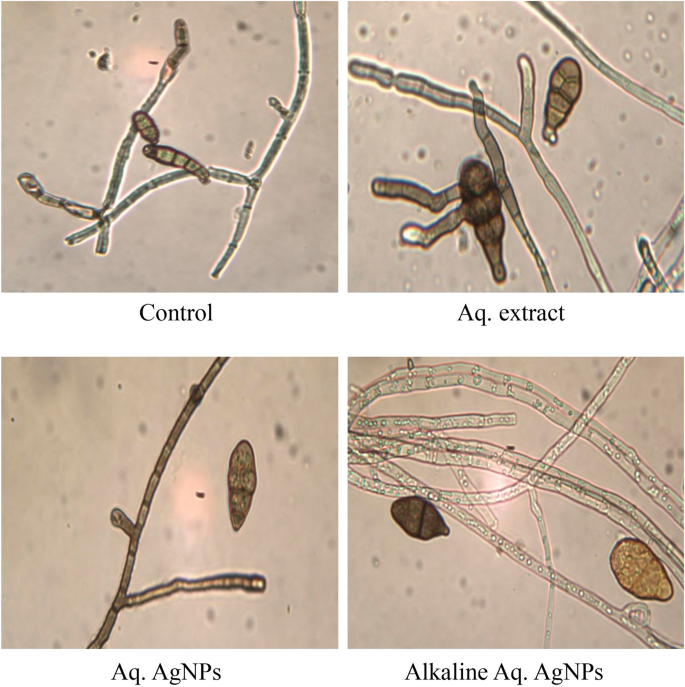
The light microscopic analysis of untreated M. phaseolina revealed a distinct morphology. The hyphae were hyaline, thin-walled, septate, and branched at the right angle. The microsclerotia were hyphae that were hardened with compact masses and oblong-shaped. The crude aqueous extract of R. strica didn’t induce any clear changes, whereas both of the biosynthesized AgNPs induced the thickening and rapture of hyphae, which appeared bale and hardened (Fig. 11).
The images of the untreated control of C. australiensis showed the sympodial, septate, flexuous, geniculated conidiophores with verrucose walls. The curved ellipsoidal conidia had rounded ends with four pseudosepta. The hyphae were protuberant, subhyaline, flexuous, light brown, and septate, with swollen and darker basal cells. The treatment with the crude aqueous extract of R. stricta or the Alkaline Aq. AgNPs didn’t reveal any significant changes in the hyphal structure, while the conidia didn’t appear. On the other hand, Aq. AgNPs induced extensive changes, where the hyphae were shorter, condensed, darker, and tighter than the control. The conidia appeared darker and raptured (Fig. 12).
Finally, the microscopic analysis of A. alternata showed that the untreated fungus had clear morphology for both conidia and hyphae. The conidia were reddish-brown, ellipsoidal with a cylindrical beak, septate with 3–4 pseudosepta, and had verrucose smooth walls. The hyaline hyphae were multicell, septate, and branched. Different treatments of R. stricta caused the conidia to be more swollen, darker (in the case of crude extract and Aq. AgNPs), non-septate (in the case of Alkaline Aq. AgNPs), and oval to circular-shaped. The hyphae were more bale, transparent, and granulated in the species treated with the Alkaline Aq. AgNPs of R. stricta (Fig. 13).
In the current study, we employed SEM to explore the morphological properties of the some of the susceptible species to the treatments under consideration. We show the species of D. halodes, D. tetramera, and C. australiensis here. The hyphae were deformed after treatment with Alkaline Aq. AgNPs, while the spores resembled the untreated control. After treatment with the aqueous extract and both biogenic AgNPs, there was an obvious contraction in hyphae and deformed spores of D. halodes. When compared to the natural structure exhibited in the untreated sample, these deformed spores had irregular forms and smaller hyphae (Fig. 14a). Without treatment, the D. tetramera control sample revealed the fungus’s original structure. However, significant hyphae deformation was seen after treatment with the aqueous extract and both biogenic AgNPs. When treated with Aq. AgNPs, the fungal spores were deformed relative to the control and were essentially missing (Fig. 14b). C. australiensis control showed the fungus in its optimum form without treatment. Significant damage was detected after treatment with R. stricta aqueous extract and biosynthesized AgNPs. The hyphae appear to be cemented together, which might be explained by the fungus’ capacity to make hydrophobic glue as a way of resistance to treatment. In comparison to the control, the detected spores look deformed and abundant (Fig. 14c).