Jan 04, 2024
Development of a unique high-performance photoelectrode using zinc oxide nanopagoda arrays and silver nanoparticles for efficient sunlight absorption and enhanced photoelectrochemical properties.
The zinc oxide nanopagoda exhibits minimal crystal defects, high electron conductivity, and step structures enabling high surface chemical reaction activity.
Introduction of silver nanoparticles improves visible light absorption, enhancing photocurrent by approximately 1.5 times, beneficial for photoelectrochemical water splitting.
The unique structure of zinc oxide nanopagoda efficiently captures ultraviolet rays, contributing to improved photoelectrochemical properties.
Ongoing research focuses on structural control and surface decoration of zinc oxide nanopagodas to increase durability and optimize hydrogen production from water splitting.
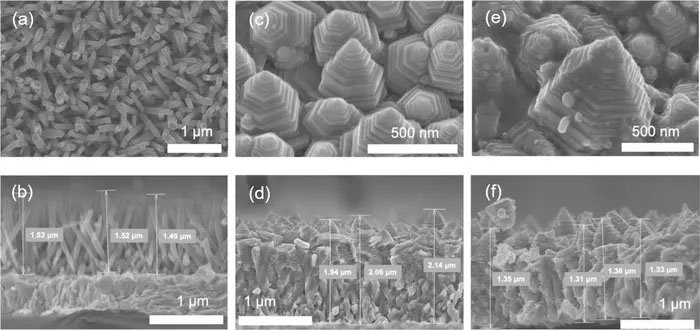 (a)(b): zinc oxide nanorod array, (c)(d): zinc oxide nanopagoda array, (e)(f): silver-nanoparticle-decorated zinc oxide nanopagoda array. The upper row includes surface images, and the lower row includes corresponding cross-sectional images. (Image: Toyohashi University of Technology)
(a)(b): zinc oxide nanorod array, (c)(d): zinc oxide nanopagoda array, (e)(f): silver-nanoparticle-decorated zinc oxide nanopagoda array. The upper row includes surface images, and the lower row includes corresponding cross-sectional images. (Image: Toyohashi University of Technology)
(Nanowerk News) A research team consisting of members of the Egyptian Petroleum Research Institute and the Functional Materials Engineering Laboratory at the Toyohashi University of Technology, has developed a novel high-performance photoelectrode by constructing a zinc oxide nanopagoda array with a unique shape on a transparent electrode and applying silver nanoparticles to its surface. The findings have been published in Electrochemistry Communications (“Ag nanoparticles decorated ZnO nanopagodas for photoelectrochemical application”).
Key Takeaways
 (a)(b): zinc oxide nanorod array, (c)(d): zinc oxide nanopagoda array, (e)(f): silver-nanoparticle-decorated zinc oxide nanopagoda array. The upper row includes surface images, and the lower row includes corresponding cross-sectional images. (Image: Toyohashi University of Technology)
(a)(b): zinc oxide nanorod array, (c)(d): zinc oxide nanopagoda array, (e)(f): silver-nanoparticle-decorated zinc oxide nanopagoda array. The upper row includes surface images, and the lower row includes corresponding cross-sectional images. (Image: Toyohashi University of Technology)

