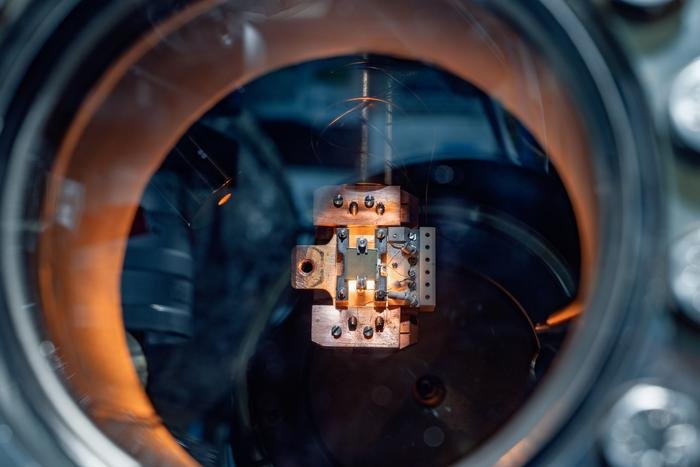
image:
Lars Mohrhusen’s junior research group aims to develop new catalysts for the conversion of carbon dioxide. The catalyst samples (like the square grey plate in the middle of the holder) are examined in vacuum chambers using various analytical methods.
Credit: University of Oldenburg / Marcus Windus
Harnessing the power of the sun to convert carbon dioxide into useful chemicals is the goal of a new junior research group at the University of Oldenburg. The international team led by chemist Dr Lars Mohrhusen will adopt a double-sustainable approach: it aims to develop precious-metal-free catalysts that use sunlight to chemically activate this relatively inert greenhouse gas. The Federal Ministry of Education and Research (BMBF) has approved 2.6 million euros in funding for the project Su2nCat-CO2 over the next six years as part of its funding programme SINATRA (for junior research groups focused on “Artificial Photosynthesis” and the “Use of Alternative Raw Materials for Hydrogen Production”).
Commenting on the project, Prof. Dr Ralph Bruder, President of the University of Oldenburg, said: “The new junior research group’s work is aimed at finding inexpensive and durable materials to replace the precious metal catalysts currently in use. The BMBF’s funding commitment acknowledges the University of Oldenburg’s extensive interdisciplinary expertise in the fields of catalysis and nanomaterials and underlines the great importance of this research for society.”
Catalysts without precious metals
Mohrhusen and his team will focus on developing catalyst materials based on readily available and inexpensive components such as titanium dioxide. The aim is to find highly energy-efficient ways to convert the greenhouse gas carbon dioxide into compounds such as methanol, formaldehyde or ethylene which can be used by the chemical industry in the manufacture of plastics or synthetic fuels, for example. “The conversion of substances like carbon dioxide generally involves precious-metal-containing catalysts, which often require high pressure and high temperatures during operation,” Mohrhusen explains. In addition to the large amounts of energy required to create the right conditions to trigger a reaction, these materials often have the disadvantage of being expensive and not particularly durable. Impurities in the gas feed, for example, can easily “poison” the catalyst material so that it becomes less active over time, the chemist points out.
Mohrhusen’s team plans to investigate two different types of hybrid catalyst materials in model systems. For this they will create combinations of titanium dioxide and semi-metal nanoparticles as the first class of materials, and organic structures on oxide surfaces as the second. In the next step, the researchers will use various techniques to characterise the systems at the atomic level– a process which typically requires ultra-high vacuum conditions. Both material classes will be photocatalysts, meaning that they become catalytically active when exposed to light. Their exposure to sunlight generates charge carriers – negatively charged electrons and positively charged “vacancies”, so called “holes” – which can then react chemically with carbon dioxide. “On the basis of these model catalysts we aim to gain a detailed, atomic-level understanding of which material properties enhance the reactivity as well as the stability of these systems,” says Mohrhusen. This can be very difficult under the technical conditions that prevail in large reactors, he explains.
Tests in microreactors
In a third sub-project the team plans to design micro reactors to test the model catalysts under more realistic conditions. This will involve bringing the catalyst materials into a gas atmosphere – a combination of carbon dioxide, hydrogen and water, for example – in a special chamber and simultaneously irradiating them with light. The researchers will analyse the formation of reaction products during the process and can also examine the catalyst materials for structural changes caused by the reaction once the tests have been completed.
Mohrhusen studied chemistry at the University of Oldenburg, where he earned his bachelor’s degree in 2014 and his master’s in 2016. He also completed his PhD in Oldenburg in 2021, in the Nanophotonics and Surface Chemistry group led by Prof. Dr Katharina Al-Shamery. As a postdoc, he has spent around three years in total conducting research at Harvard University (USA) and Aarhus University (Denmark).
The Friedrich-Alexander-Universität Erlangen-Nürnberg, Leiden University (the Netherlands), Aarhus University (Denmark), the University of Florida (USA) and the two companies Evonik and Leiden Probe Microscopy are supporting Mohrhusen’s project as associated partners. The BMBF’s funding line for junior research groups enables outstanding early-career researchers to set up their own research groups and work on innovative projects while advancing their careers on the path to a professorship or other leading academic positions.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

